content

手性科学 (chirality science)
超分子化学 (supramolecular chemistry)
胶体界面化学 (colloid and interface chemistry)
光化学与物理 (photochemistry and photophysics)
纳米化学 (nanochemistry)
纳米光子学 (nanophotonics)

1. 分子/纳米组装体系的光化学与物理研究 (圆偏振发光, 上转换发光, 上转换圆偏振发光) (Photochemical and photophysical Studies of Molecular/nano-assembled Systems (Circularly polarized luminescence, upconverted luminescence, upconverted circularly polarized luminescence))
2. 手性光电材料与器件相关成果 (Chiral optoelectronic materials and devices)
3. 光子频率上转换材料的开发及其应用拓展 (Development and application of upconversion materials)
4. 软物质体系手性科学问题 (Science of the chirality in soft matter systems)

Photon upconversion (UC), the conversion of low-energy light into higher-energy emission, has a wide variety of applications in the fields of photodynamics therapy solar, cell energy conversion, bioimaging, photocatalysis. Generally speaking, UC requires two or more low-energy photons to produce one high-energy photon. Whereas, another interesting UC phenomenon can be achieved by exciting vibrational-rotational energy levels of ground state to the first level of the excited state, which is called hot-band absorption-based single-photon upconversion (HBA-UC).
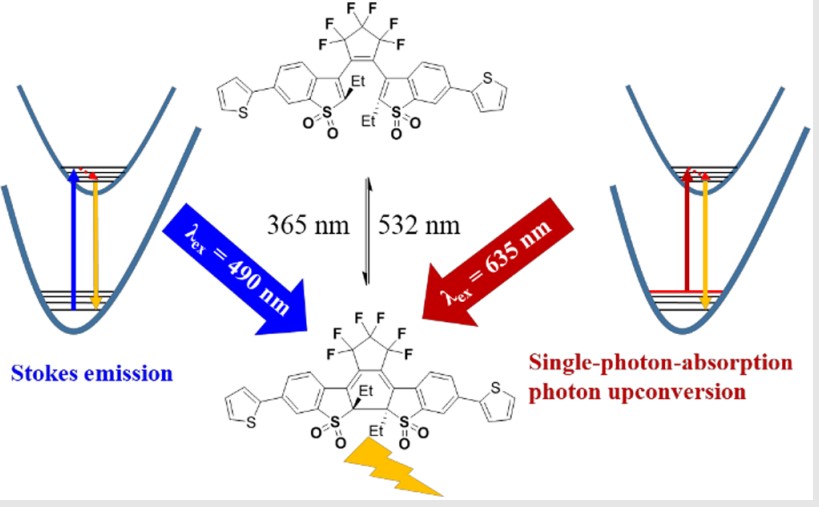
In 2019, for the first time, we studied a series of diarylethene (DEAS) molecules with HBA-UC properties. When irradiated by 365nm light, DEAS would experience a ring-closing process followed by an increase of emission intensity, while a ring-opening process and a decrease of emission intensity when irradiated by 532nm light. According to the UV-Vis spectra, the closed-ring state of diarylethene molecules had almost no absorption around 635nm but exhibited strong UC emission when excited by a 635nm laser. Linear proportionality between emission intensity and the laser power indicated the single-photon absorption process. Because of the low energy of the excitation light, higher vibration-rotation levels of ground states were needed during the transition process, which means that hot band absorption is connected with the UC process. The populations of the vibration-rotation levels of the ground state were determined by a Boltzmann distribution, which is a temperature-dependent phenomenon. The temperature-dependent HBA-UC emission was investigated from 200K to 300K. We found that with the temperature increase, the emission intensity gradually increased, which further proved the HBA-UC process. Furthermore, a large quantum yield (13.5%) was observed here. Although the absorption is low, UC emission was observed directly by naked eyes.
In 2021 we synthesized nine DAES derivatives with different electron-donating nature of the substituent groups which would change the intramolecular charge transfer (ICT) between benzothiophene 1,1-dioxide core and substituent groups. We found that the strong ICT feature of DAES was responsible for the wide full width at half-maximum (FWHM) of absorption spectra, which enriched the molecules’ distribution of high-vibrational energy levels in the ground state and further improve the HBA-UC characteristics. Besides, solvents and the acid-base system could regulate the HBA-UC emission peak, providing new methods for optical devices and biology.
reference
CCS Chem, 2020, 2, 665–674
Adv. Optical Mater, 2021. 2102180

Triplet–triplet annihilation (TTA) based photon upconversion is a method to convert two low-energy photons to a high-energy photon with relatively high efficiency. TTA-UC systems typically include two parts: an energy harvester/triplet sensitizer and a triplet annihilator/emitter. The sensitizer absorbs low-energy photons and transfers the harvested energy to the annihilator through non-radiative energy transfer (ET). Subsequently, two annihilators in their triplet state can encounter yielding one singlet ground state and an excited singlet state with higher energy than the absorbed photons, then the anti-stokes emission is generated. However, the low singlet generation efficiency of the annihilators prevents the improvement of the TTA-UC efficiency.
In 2021, we designed and synthesized a fluorescent bichromophoric perylene derivative (PCP) as a triplet annihilator which exhibited brilliant TTA-UC property by coupling with a suitable metallic-porphyrin type sensitizer (PtTPBP). Compared with perylene as triplet annihilator in the previous report, PCP had similar triplet energies, but a higher molar extinction coefficient. Then we found that with the concentration of PCP surpassing 10-4 M, the emission yield gradually decreased because the evanescent collisional complexes were formed, but this decrease was less pronounced than perylene, allowing no critical energy losses with high dye concentrations. Under 20 W/cm2 excitation power, maximum upconversion output quantum yield (QYout) measured was 0.42 ± 0.03 for a PCP: PtTPBP (5 × 10-4 M: 10-5 M) solution in THF, which is the highest QYout performance observed for TTA-UC.
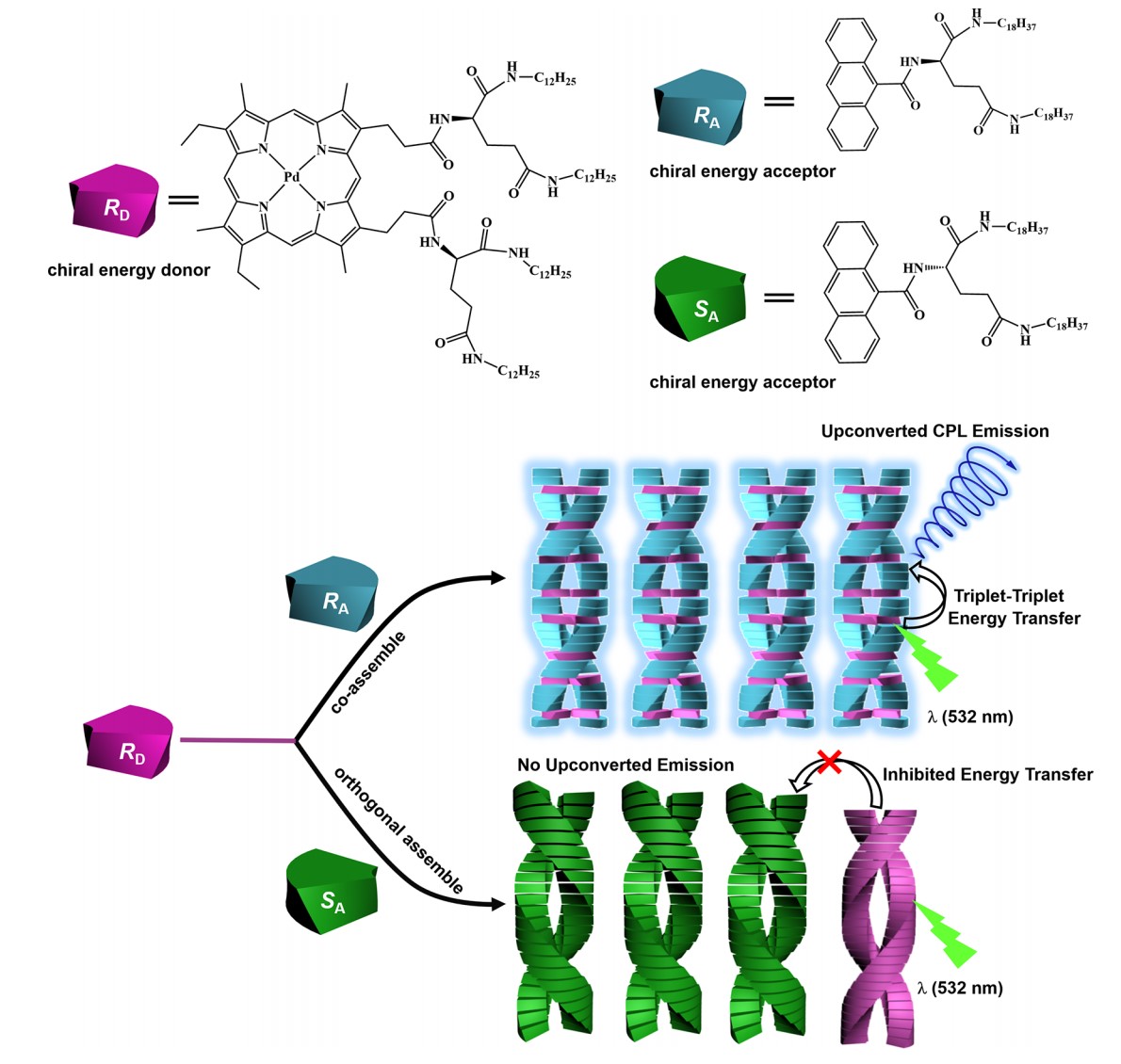
At the same time, chiral sensitizer-based enantioselective TTA-UC was achieved in our research. In this work, chiral glutamic scaffold modified sensitizer (RD) and anthracene-derived chiral acceptor (RA/SA) were used here. The chiral gelator moiety N, N’-bis(dodecyl)-L(D)-amine-glutamic diamide at their molecular skeletons endowed them with perfect self-assembly ability. Interestingly, when RD assembled with RA, high performance TTA-UC could be achieved, while poor TTA-UC when assembled with SA. The CD spectra and UV−vis measurements revealed that RD coassembles with RA but orthogonally assembled with SA. After irradiating with 532nm laser, RA/RD gel and SA/RD gel showed different upconversion intensity, because the donor-acceptor distance in segregation SA/RD system is longer than the distance of electron exchange based Dexter mechanisms, resulting in inhabitation of triplet-triplet energy transfer.
reference
J. Am. Chem. Soc, 2021, 143, 13259−13265
J. Mater. Chem. C, 2021.9 14201–14208

Chiral substances play an important role in medicine industry, living systems, and chemical reactions. In addition, some chiral compounds with chromophores can spontaneously emit circularly polarized light with no need for linear polarizers and quarter-wave plates. This phenomenon is called circularly polarized luminescence (CPL). CPL has broad application in 3D imaging, information encryption and transmission, and even disease detection. To evaluate the degree of CPL, the dissymmetry factor (glum) is defined as:

where IL and IR are the intensities of left- and right-handed light, respectively. Nowadays, how to obtain large glum is an important issue for practical application of CPL materials. Recently, a chiral host-achiral guest method was proposed in our research.
(1) Micelles

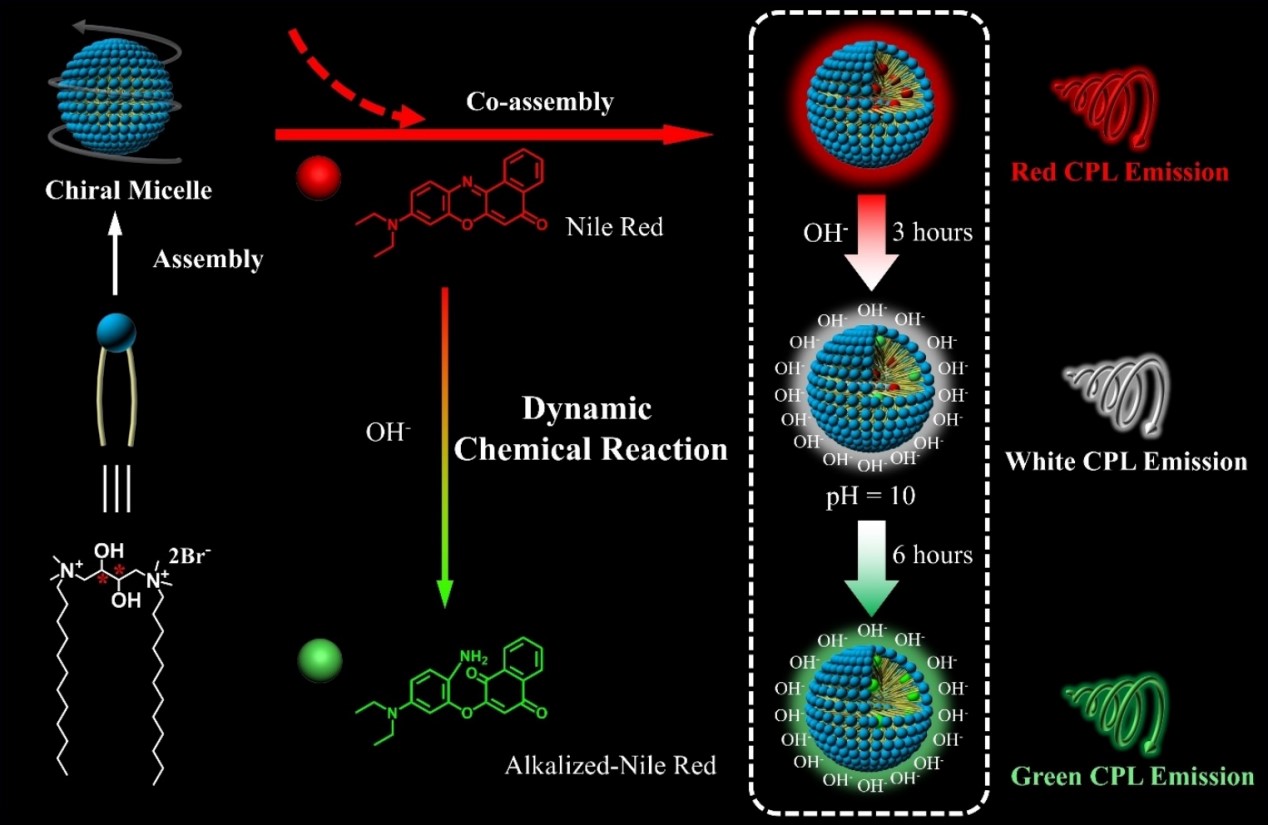
When the concentration of surfactants surpasses the saturation concentration in aqueous solution, the excess surfactants will assemble into micelles, whose hydrophilic group is outward and the hydrophobic group is inward. This character enabled us to endow both hydrophilic and hydrophobic emitters with CPL properties. In 2022, we used chiral 1,4-bis (dodecyl-N, N-dimethylammonium bromide)-2,3-butanediol (GS) as surfactants and added both hydrophilic and hydrophobic dyes into the micelles at the same time. We found that chirality transfer induction occurred in both surface and interior of the micelles through independent processes. Besides, we first realized the modulation of excited state chirality of a dynamic chemical reaction in the chiral confined space. Nile Red molecules (NRs), a kind of hydrophobic emitter whose “C=N” bonds could be broken in alkalized aqueous conditions and emission peak would be changed. So that, the CPL experienced a stepwise change from red to white to green, illustrating the modulation of the excited state chirality by a dynamic chemical reaction.
reference
Angew. Chem. Int. Ed, 2022, 61, e202115600
(2) Gels

Self-assemble refers to the spontaneous formation of ordered structures from building blocks with the help of non-covalent bonds, such as hydrogen bond, van der waals forces, halogen bond, etc. Supramolecular self-assembly has become a universal method to construct CPL materials. In this way, the complex hierarchical chiral organization processes and chiral structures can be built rapidly to get a higher degree of chirality with minimal synthetic effort, and through co-assembly process, achiral molecules can be involved in fabricating a chiroptical system. Through the chirality transfer process, the resulting assembly could easily induce or amplify the chirality of chromophores. Today, supramolecular gels are widely used as chiral host matrixes to endow both organic and inorganic emitters with CPL activity.
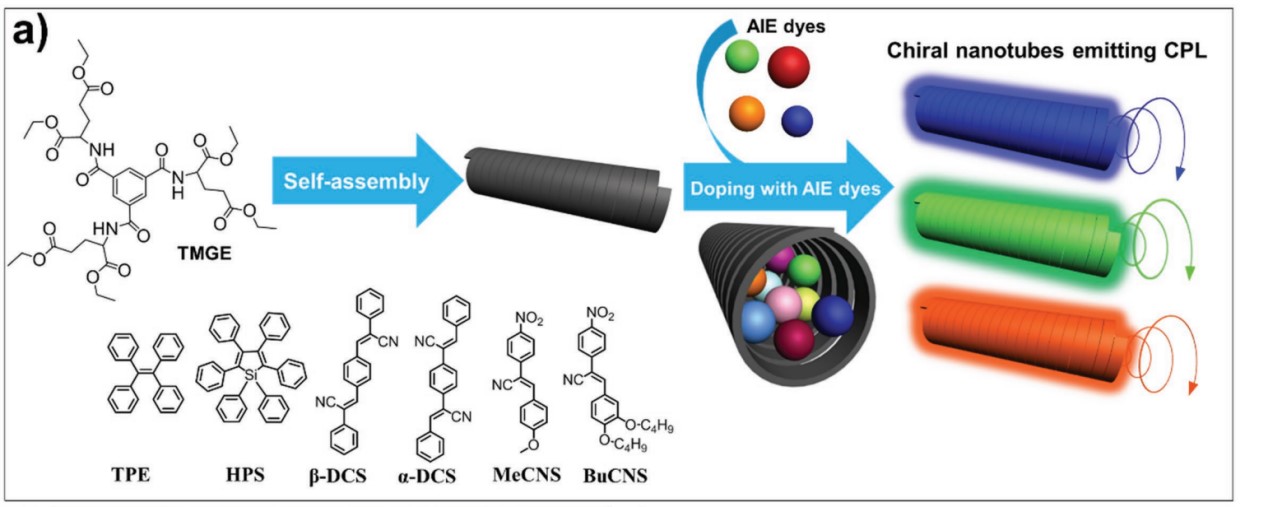
In 2017, we have developed a general method for constructing CPL-active materials by encapsulating achiral aggregation-induced emission (AIE) dyes into the chiral space of supramolecular gel nanotubes. During the formation of gel, the chirality transfer from host chiral space to achiral AIE guest induced the chirality of the achiral components, the calculated value of glum of the CPL signal is about 1.3 × 10−3. Because of the AIE effect, the luminescence intensity increased simultaneously in the gel.
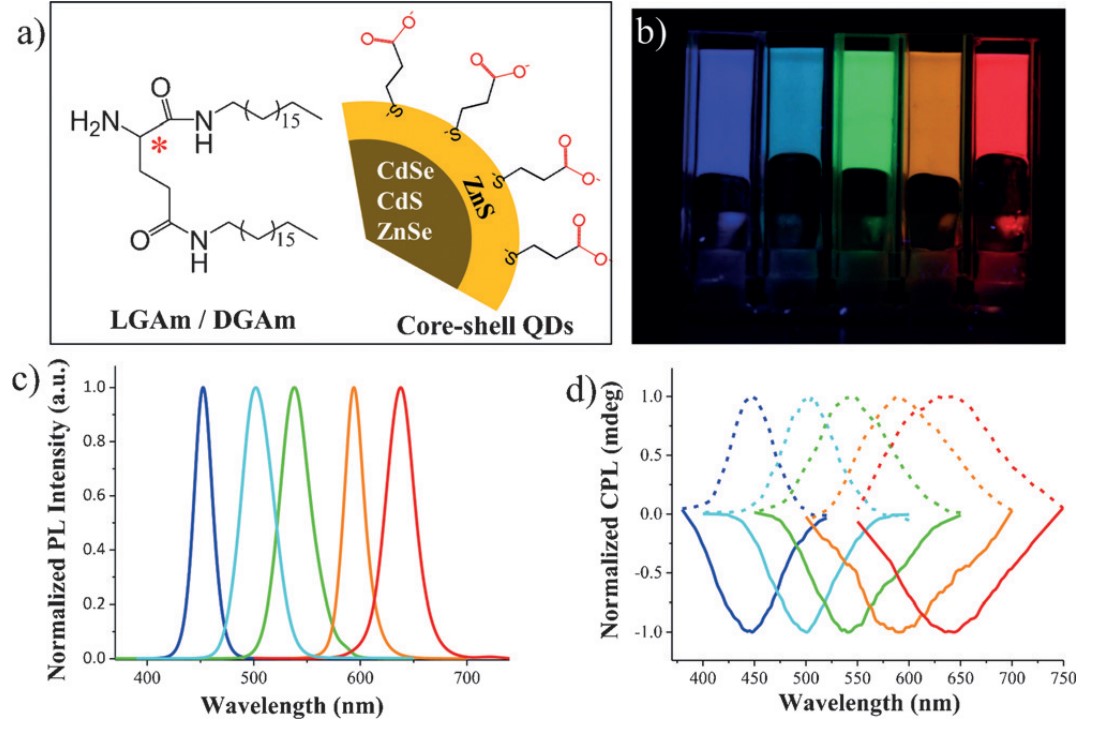
Inorganic quantum dots (QDs) could also exhibit CPL properties through this method. We coassembled core-shell-type QDs with organic lipid gelator N, N’-bis(octadecyl)-L(R)-glutamic diamide. Attribute to the electrostatic and hydrogen-bonding interactions between the capping reagent of the QDs and the amine groups of the chiral gelator, QDs arranged along the helical gel nanotube and were endowed with chirality. But long spacer length of capping reagents between QDs and gels will prevent chiral induction process. Besides, by tuning the type or the blending ratio of QDs, we fabricated QDs with full-color and white CPL materials. The glum we got here had the same order of magnitude: 10−3.
With this supramolecular gelation method, perovskite nanocrystals (NCs) could also be endowed with CPL ability. Perovskite NCs were synthesized by a polar-solvent-free approach. The resulting CsPbX3 (X = Cl, Br, I, Cl/Br, and Br/I) perovskite could emit different colors by varying the halide composition. Considering that the surface of the as-synthesized NCs is capped with oleic acid and oleylamine, we selected amine-containing lipid (LGAm/DGAm) for replacing some of the surface ligands and facilitating the formation of chiral co-assemblies. Mirror-image CPL signals could be gotten in various co-assemblies from 410 to 600 nm, with the maximum glum value up to 7.3 × 10−3. However, in a mixture of perovskite NCs and lipids, there was no CPL signal, which indicated that helical cogels played an important role in the achievement of CPL.
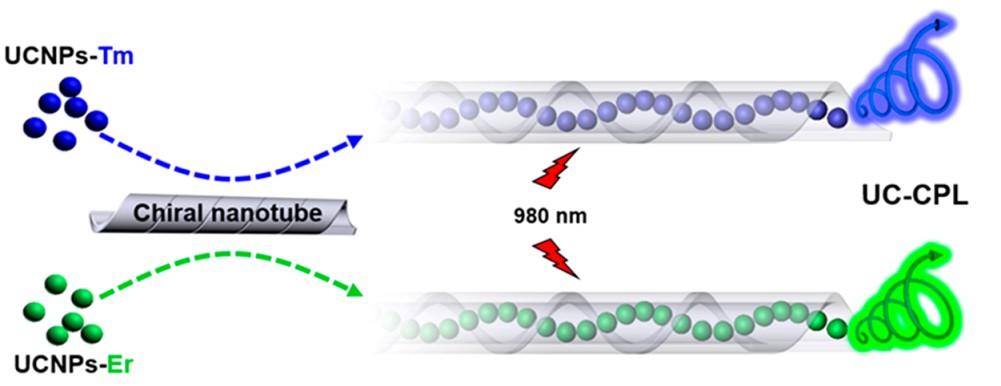
Upconversion nanoparticles (UCNPs) were another prominent inorganic luminescent nanomaterials, which could also be endowed with CPL properties in LGAm/DGAm nanotube. In our current work, two kinds of UCNPs were successfully helically encapsulated into chiral nanotubes. Similarly, the assembly exhibited strong CPL signal in all emission peaks of two kinds of UCNPs (average glum was 5.48 × 10-3), and kept CPL silence in a non-assembly system.
reference
Adv. Mater, 2017, 295, 1606503
Angew. Chem. Int. Ed, 2017. 56, 12174 –12178
Adv. Mater, 2018. 30, 1705011
ACS Nano, 2019. 13, 2804−2811
(3) Liquid crystals

Liquid crystals can be induced into chiral nematic liquid crystals (N*LCs ) and form a stop band by adding chiral molecular. Generally, there are two mechanisms of CPL in N*LCs. When the stop band is far away from the emission peak, the obtained CPL is transmitted along the helical axis of the N*LC getting the polarized direction same as the handedness of the N*LC. When the stop band is matching with the emission peak, the circularly polarized light with the same handedness will be reflected while the one with opposite handedness will be transmitted, resulting in relatively high glum but low emission intensity. For the CPL research field, N*LCs have gained more attention because they have unique optical properties of generating CPL with an incomparably high glum value, they are remarkably general for almost all emitters and they perform excellent stimulus responsiveness.
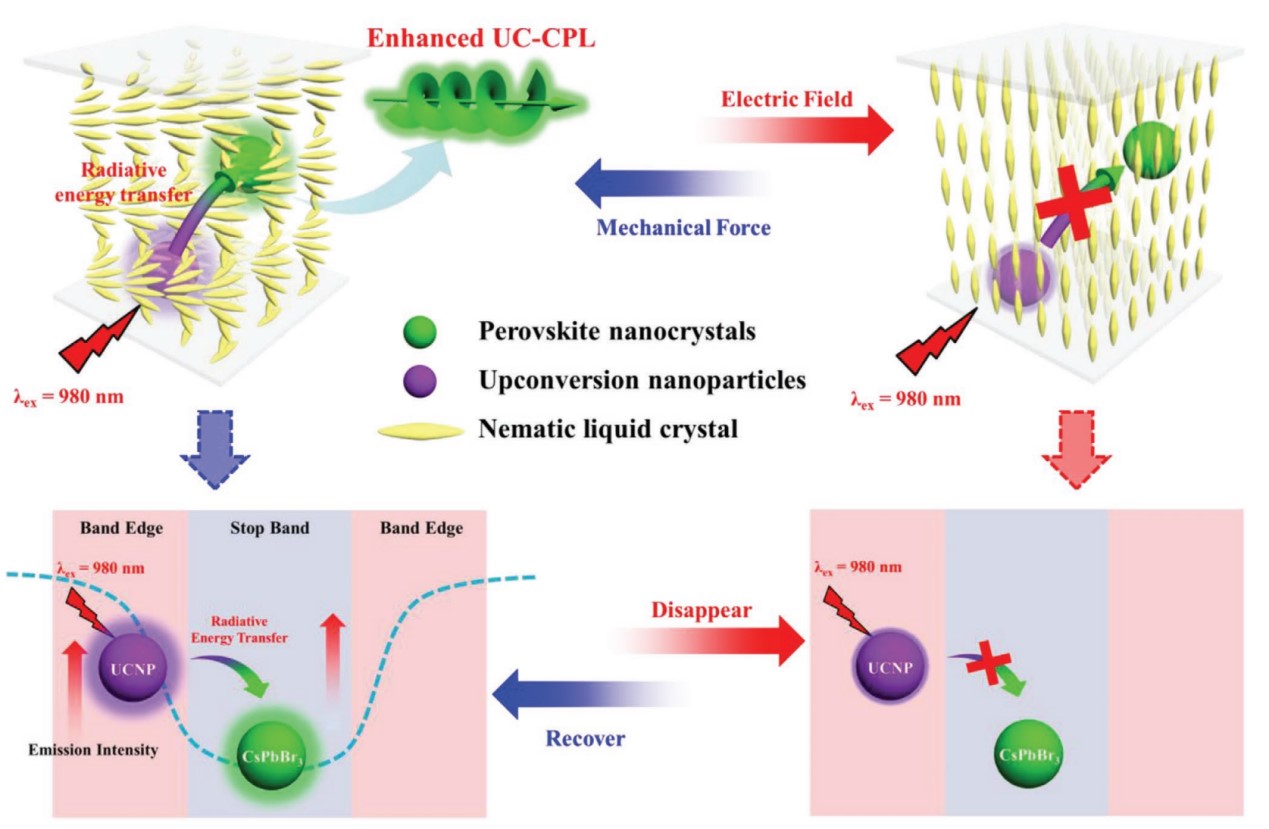
In 2020, we added CsPbBr3 perovskite nanocrystals and upconversion nanoparticles (UCNPs) into commercial N*LCs, and regulate the stop band by chiral molecular S811. In this system, the energy was transferred from UCNPs to perovskite nanocrystals. The emission peak of UCNPs was located at the center of the stop band while the emission peak of perovskite nanocrystals was located at the edge. In this way, band edge enhanced CPL was gotten with the glum arriving 1.1. Moreover, the CPL signal can be controlled by the electric field and mechanical force, realizing a voltage-force-regulated switch of UC-CPL.
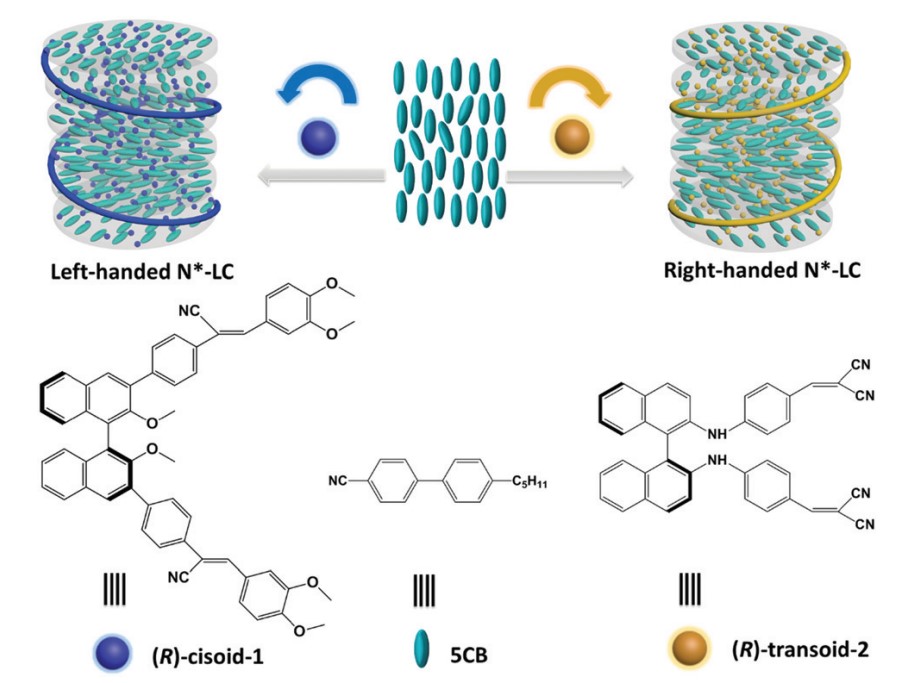
In 2019 a kind of chiral binaphthyl derivatives substituted at 3,3’ positions or 2,2’ positions of binaphthyl rings, abbreviated as R/S-1 and R/S-2, were used as both chromophores and chiral dopants for N*-LCs. Interestingly, the chiral binaphthyl derivatives with the same (R)-configuration could induce different chiroptical properties of the N*-LCs, which was assigned to the different dihedral angles of binaphthyl derivatives. N*-LCs induced by R-1 exhibited negative CPL signals, while N*LC induced by R-2 exhibited positive CPL signals, the glum were around 0.25 and 0.27, respectively.
reference
Chem. Commun, 2019, 55, 5914--5917
Adv. Mater, 2020, 32, 2000820
(4) Perovskite nanocrystals

Perovskite nanocrystals are attracting great interest due to their excellent photonic properties. One of the main advantages of these materials is the fact that the optical band gap can be easily tuned by exchanging individual components. Hot-injection method is a universal approach to synthesize perovskite, but high temperature will result in racemization of chiral amine molecules. Fortunately, though chiral-ligand-assistant method by ultrasound, we successfully endowed perovskite nanocrystals with CPL property.
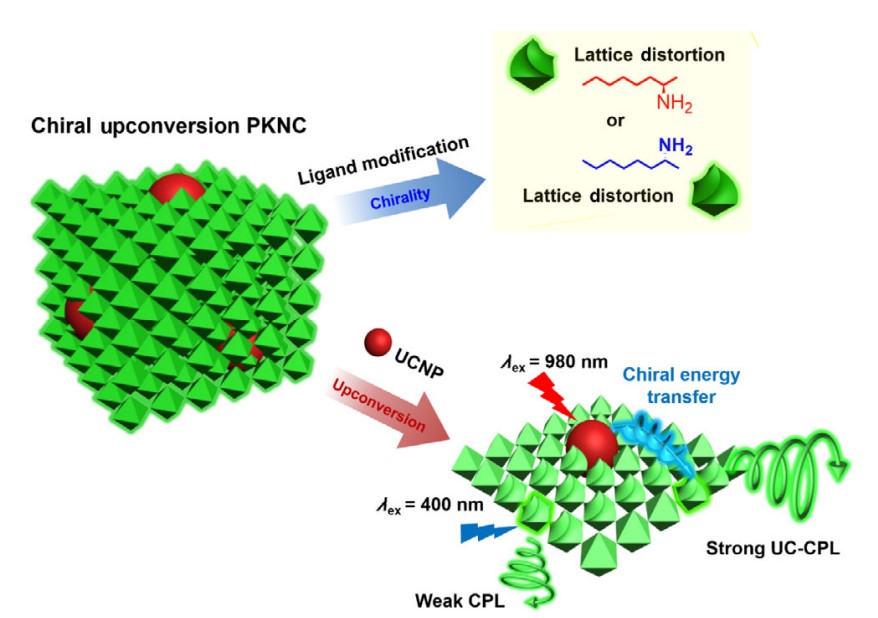
In 2022, we applied a polar-solvent-free approach to synthesize chiral perovskite nanocrystals, and we gained UCNPs@PKNC system by adding UCNPs in precursor solutions. In this system, chirality transferred from capping ligands to perovskite nanocrystals and UCNPs, and glum was further amplified to 4.5×10-3 through the energy transfer from UCNPs to perovskite nanocrystals.
reference
Nano Research, 2022, 15, 1047–1053
(5) Metal-organic frameworks

Porous crystalline nanomaterials (PCNMs) include metal-organic frameworks (MOFs), metal-organic cages (MOCs), covalent organic frameworks (COFs), etc. PCNMs can be assembled with the corresponding organic and inorganic components, providing a powerful solution for precisely controlling the arrangement of building blocks. Moreover, PCNMs have applications as host matrices owing to their porosity, variable pore size, and ultrahigh surface area. Such features enable PCNMs to enhance certain properties in the solid-state. For instance, ordered structures with high crystallinity can suppress the aggregation-caused quenching effect of luminophores. By endowing PCNMs with chirality, exceptional properties could be obtained, so PCNMs are widely investigated for their chiroptical applications.
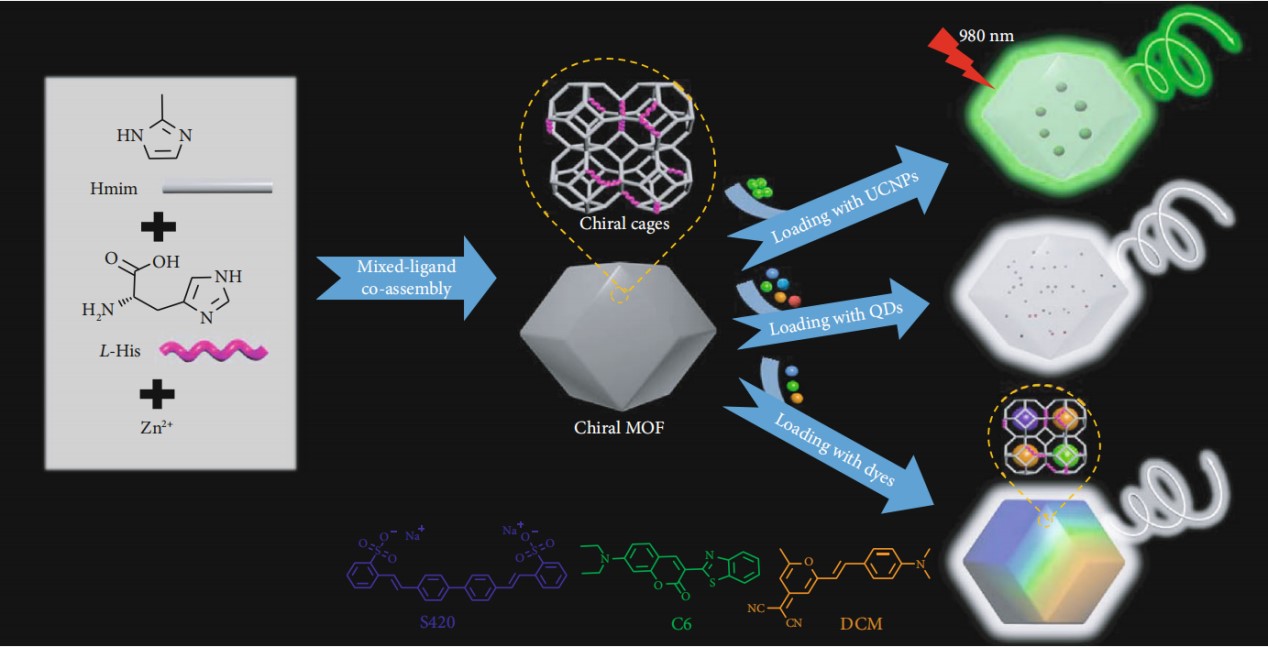
Compared with other porous materials, MOFs have special applications as host matrices due to their unique features, including high crystallinity, regular and tunable structures, ultrahigh porosities and specific surface areas, and tunable pores. In our work, we utilized a facile and economically feasible strategy to fabricate the chiral zeolitic imidazolate framework (ZIFs) by incorporating L-/D-histidine (His) into the ZIF-8 framework using a mixed-ligand coassembly pathway. Achiral dyes, quantum dots (QDs), and upconversion nanoparticles (UCNPs) were easily loaded into the chiral ZIFs during the synthetic process, and achieved solid-state CPL-active materials with high luminescence efficiency. The emitting colors can be flexibly tuned by changing the categories of dyes and QDs. In addition, white light CPL emitting MOF materials are obtained by introducing various dyes or QDs simultaneously. Meanwhile, UC-CPL with a high dissymmetric factor(1.2×10-2) is achieved through loading UCNPs into the chiral MOF matrixes. It was found that chiral ZIFs in this work could endow chirality to the organic dyes which dispersed in cages by chiral confined space or environment. But QDs were too large to be encapsulated into these chiral cages, which were distributed into the framework, and surrounded by the chiral ZIFs showing anticlockwise polarization as a consequence.
reference
Research, 2020, 12, 6452123

Although there are many methods to design the CPL materials, the glum is also relatively small for the application. In our research, we developed numerous methods to further amplify the glum, giving new approaches for future construction of CPL materials.
(1) Energy transfer enhanced circular polarization

According to the earlier research glum is determined by both the electric dipole transition moment (μgn) and the magnetic dipole transition moment (mgn). In the organic system, compared with μgn, mgn is too small to account, so the glum is mainly determined by μgn.
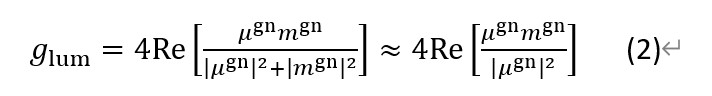
The radiationless energy transfer process will obviously decrease μgn. Based on this concept, a new method for amplifying the glum is built.
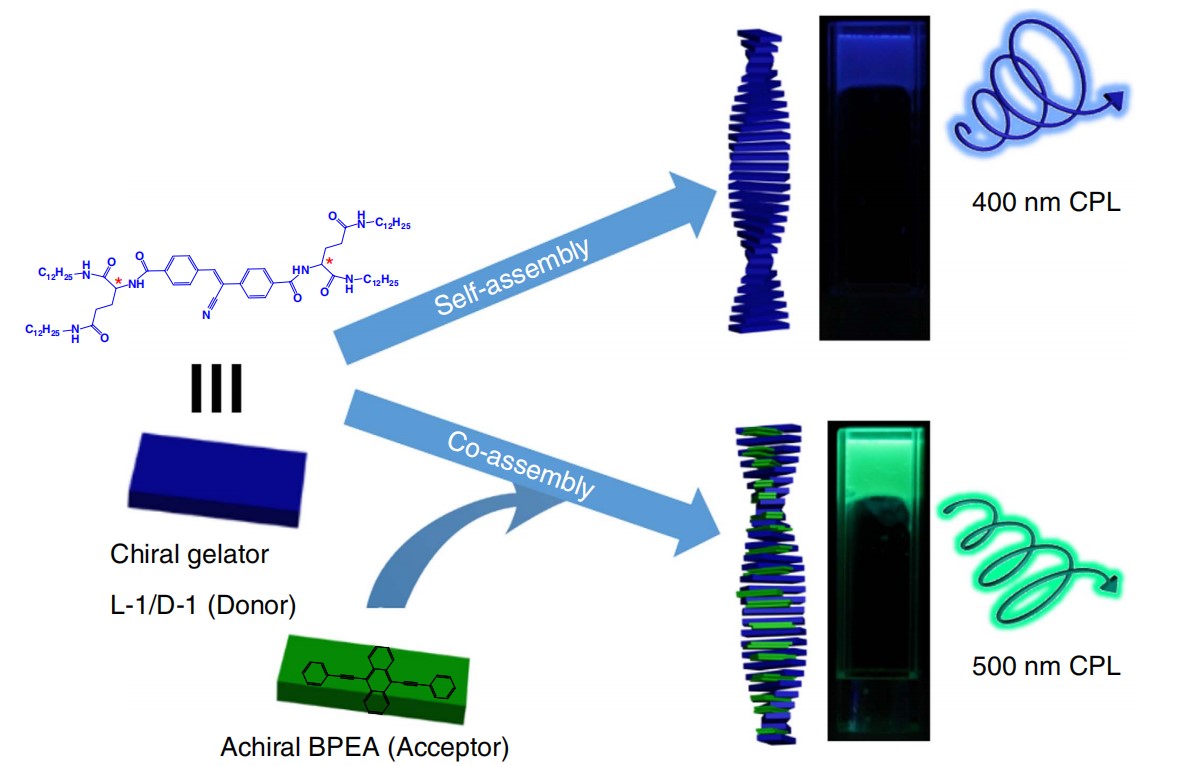
In 2017 we designed a self-assembly system based on the chiral donor and achiral acceptor. Because of the chiral confined space and environment, achiral acceptor could be induced with CPL property. Moreover, the designed gelator (L-1 or D-1 for short), which contains a donor chromophore, cyano-substituted stilbene could transfer its energy to acceptor: 9,10-bis(phenylethynyl)anthracene (BPEA). According to the measurement, attribute to the energy transfer process, glum was amplified from 1.2 × 10-3 to 3.0 × 10-3. This result proved that Förster resonance energy transfer (FRET) could significantly amplify the dissymmetric effect.
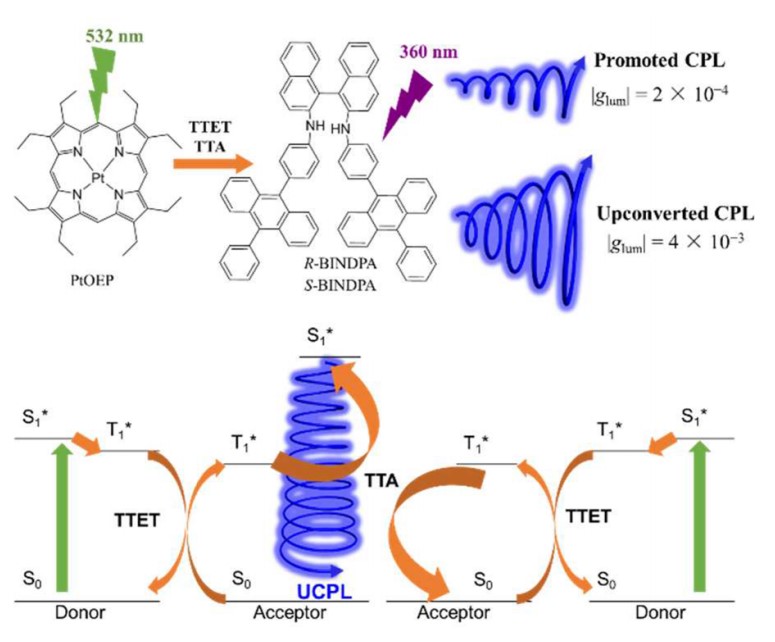
Triplet−triplet annihilation-based photon upconversion (TTA-UC) was a kind of energy transfer process, which attracted abundant attention because of its high efficiency, noncoherent excitation source tunable excitation and emission wavelengths. In 2017, we used CPL active binaphthyldiamine moiety with two 4-(10-phenylanthracen-9-yl)phenyl groups as acceptors and achiral Pt(II) octaethylporphine as sensitizers. In this way, one order of magnitude amplification of the glum in TTA-UC CPL was obtained in comparison with the normal promoted CPL, which provided a new strategy to design CPL materials with higher dissymmetry factor.
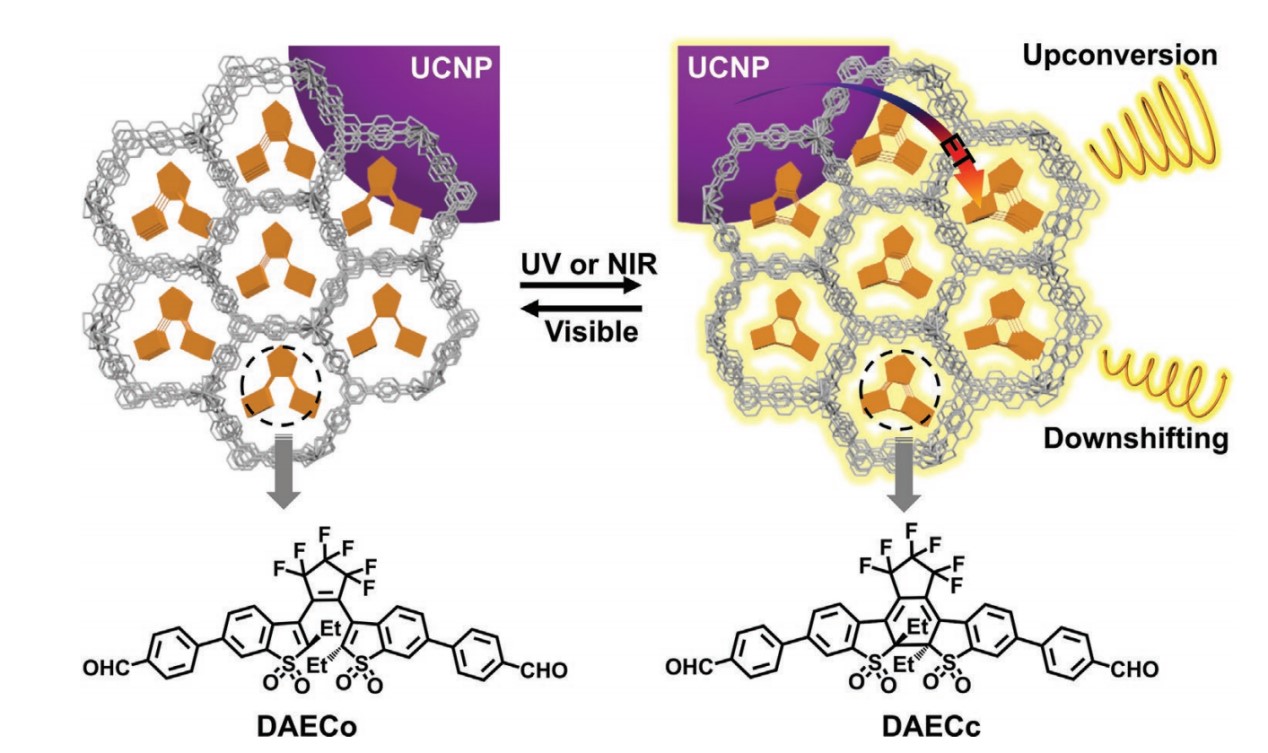
In 2021, we loaded formylphenyl-substituted diarylethene derivatives (DAEC) and upconversion nanoparticles (UCNPs) into chiral MOFs to construct a dual upconversion (UC) and downshifting (DS) CPL switch. Upon visible light irradiation, closed-ring DAECc transformed into open-ring DAECo, with the fluorescence and CPL signals being silent. Reversible photoisomerization was driven by either UV or high-power NIR light. The corresponding signals of both DS-CPL and UC-CPL were obtained under UV and NIR light excitation, respectively. Through an energy transfer process, the glum value of UC-CPL (7.8 × 10−2) showed significant amplification in comparison with that of DS-CPL (1.7 × 10−2).
reference
Nat. Commun, 2017, 8, 15727
J. Am. Chem. Soc, 2017, 137, 9783−9786
Adv. Mater, 2021, 33, 2101797
(2) Charge-transfer enhanced circular polarization

As glum is directly determined by μgn and mgn, charge-transfer (CT) complex with large mgn and small μgn would produce a large glum in the circularly polarized emission. In our work in 2019, an interesting example of a CPL-active CT complex composed of a chiral emissive donor (R/S-Py) and achiral electron acceptors (TCNB) was given. The intermolecular π-π interaction between chiral molecules resulted in dimer formation. Then, the dimer and TCNB sandwiched one another with the CT interaction to form mixed-stack packing along the c axis, which leads to the long-range ordered fibers. More surprisingly, glum value of CT complexes was calculated to be 0.017, almost 200 times larger than that of the chiral donor.

reference
Angew. Chem. Int. Ed, 2019, 58, 7013 –7019
(3) Arene–perfluororene interactions enhanced circular polarization

In our current research, we found that Arene-perfluoroarene (AP) interaction, a kind of the electrostatic interaction between a non-fluorinated and a perfluorinated aromatic ring, could significantly improve the glum. In this study, chiral emissive polycyclic aromatic hydrocarbons (PAHs) worked as arene and achiral octafluoronaphthalene (OFN) as perfluoroarene. Chiral emissive PAHs showed a relatively small glum in the amorphous state, but glum increased from 8.0 × 10-4 to 1.1× 10-2 when it coassembled with OFN.
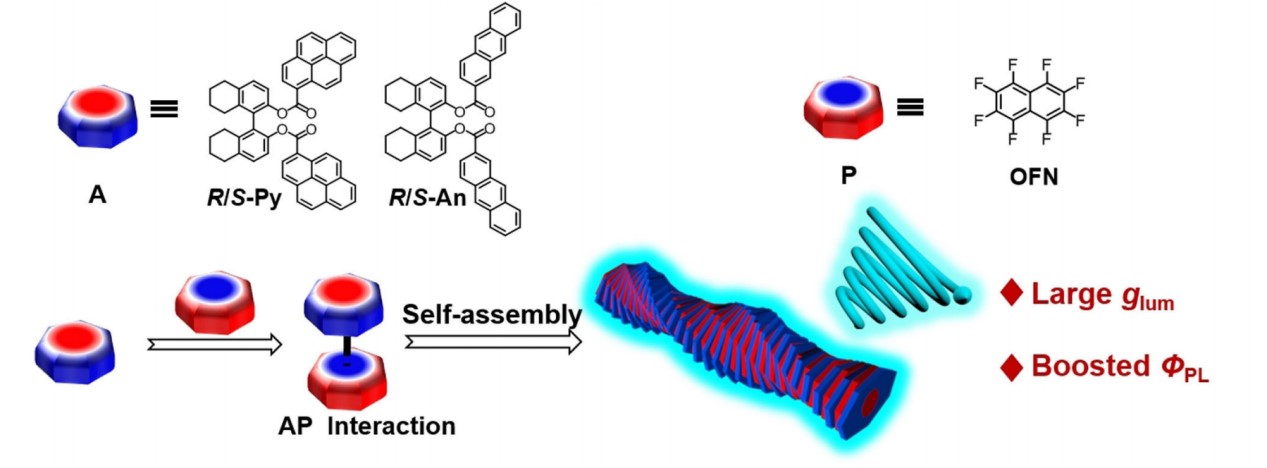
reference
Angew. Chem. Int. Ed, 2021, 60, 4575-4580
(4) Plasmon enhanced circular polarization

In earlier research, surface plasmon resonance (SPR) had been demonstrated to enhance the CPL, however they all applied it in single organic molecule systems. Molecular self-assemblies, which had strong orbital angular momentum (OAM), possessed better CPL performance. Meanwhile, as an inherent property of electromagnetic fields, spin-orbit interaction (SOI) had been widely demonstrated in experiments and theories as an efficient physical process for enhancing OAM, through a plasmon involved process. So that we used chiral emitter R-OPAn as gelator, and achiral silver nanowires (AgNWs) to provide SPR. Fixing the concentration of R-OPAn-NPs at 5 × 10−4 M, the glum value was significantly amplified with the addition of AgNWs. The maximum value could reach 1.7×10-3 (mAgNWs/mR-OPAn-NPs = 2.5), which was almost 5.0 times in magnitude compared with the glum value of R-OPAn-NPs. However, when the mixing mass ratio was over 2.5, the glum had a decrease because of the reabsorption. Moreover, the glum value of R-OPAn-NPs/AgNW composites significantly enhanced with the increase of magnetic field, which indicated that amplification could be attributed to the plasmon enhanced SOI.
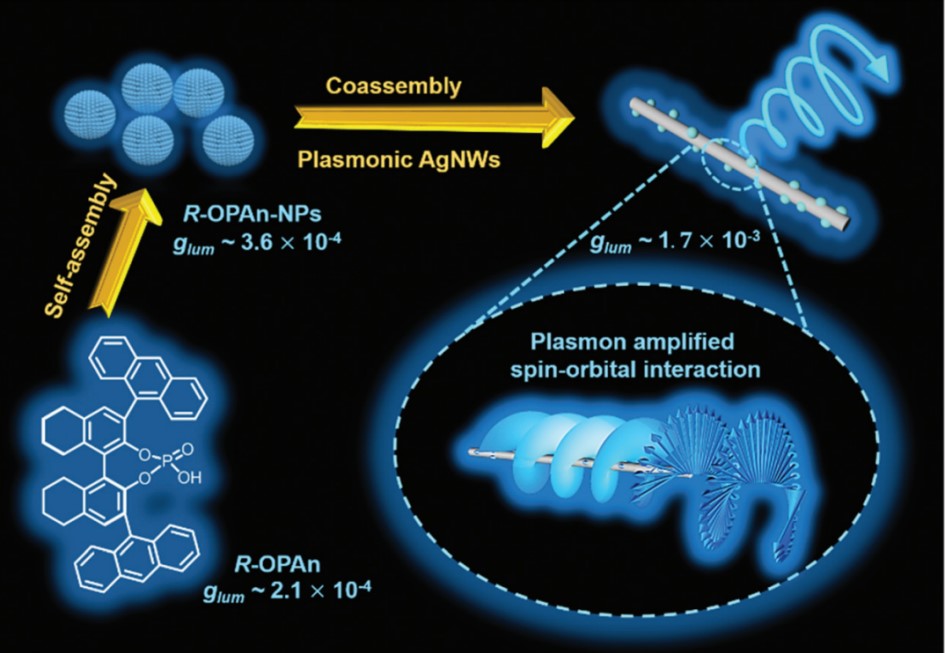
reference
Nanoscale, 2020, 12, 19760-19767

(1) Information encryption

Circularly polarized light has special polarization properties, which provide us the idea of designing the information encryption device. We explored their application using CPL liquid crystals. According to the different CPL light intensity transmitted by different circular polarizers, two detection modes could be established to input different information, and different signals could be output through the photoelectric signal modulator. Based on this, we designed a simple encryption functional signal generator system. For right-handed liquid crystals, when the fast axis of the quarter-wave plate and the light transmission axis of the linear polarizer were at 45°, a high light intensity signal was input, and a sine wave signal was displayed after modulation. On the contrary, when the fast axis and the light transmission axis are at 135°, the output was weaker and the square wave signal was displayed to achieve the effect of data encryption. The left-handed liquid crystal showed the opposite law, and the achiral liquid crystal had no encryption effect.
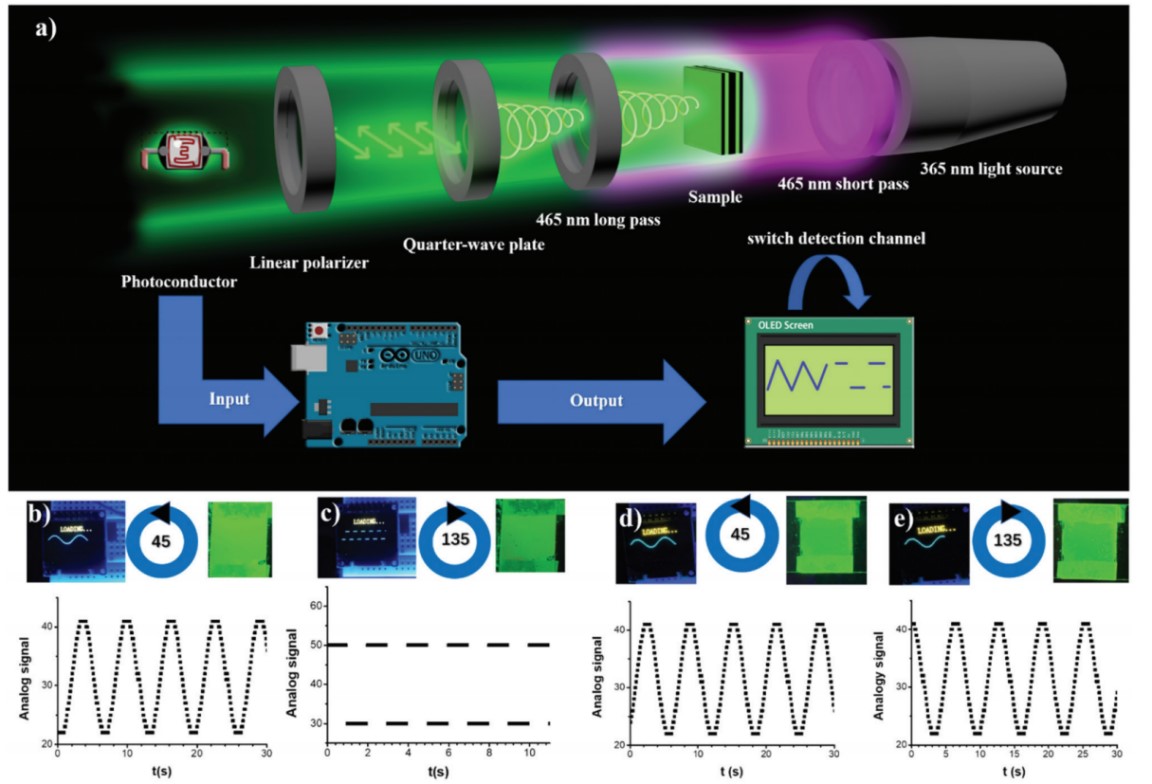
reference
Mater. Adv, 2021, 2, 3851–3855
(2) Enantioselective photopolymerization

In 2020, we introduced TTA active pairs into a chiral nematic liquid crystal, resulting in a circularly polarized ultraviolet luminescence with a large glum value. With the help of high-energy UV light, the polymerization of diacetylene can be well initiated. Moreover, due to the higher glum after sequential amplification, the system can stably control chiral polymerization. Here we repeated this polymerization ten times. All of the resulting product showed the single chirality, which was the same as annihilator, indicating that CPL could stably regulate this reaction.
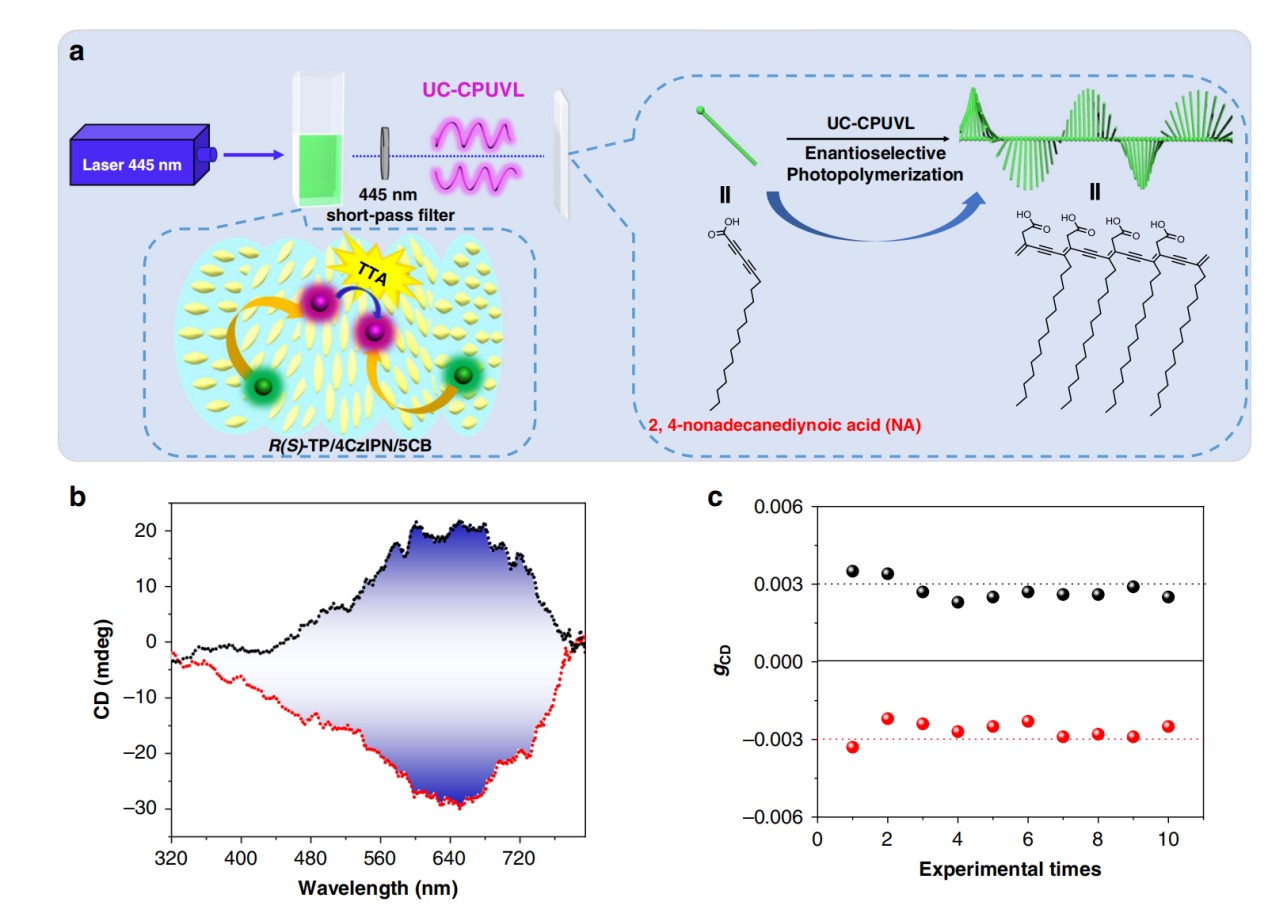
reference
Nat Commun, 2020, 11, 5659
(3) Logic circuit

Based on the fluorescence and CPL signals of chiral MOFs loaded diarylethene derivatives and upconversion nanoparticles in PMMA film in responsive to UV, visible, and NIR light, a chiral logic circuit with a 2D information output was designed. When irradiated with 365nm and 980nm light, these materials would exhibit smaller and larger glum, respectively. But if irradiated by 465nm light, DAEC would experience a close-ring process and expressed no luminescence. With three excitation lights as input signals, low-glum and high-glum circularly polarized light as output signals, a logic circuit with two-dimensional information output capability could be constructed as shown in the picture below.
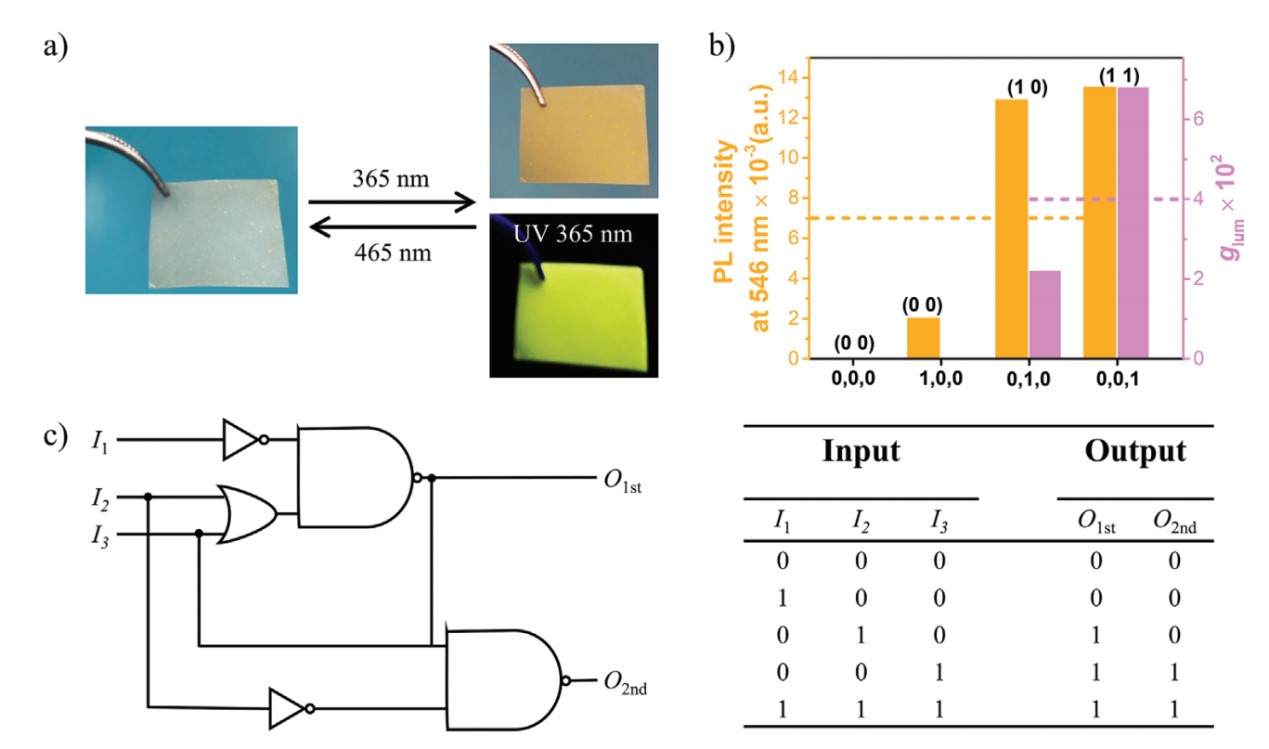
reference
Adv. Mater, 2021, 33, 2101797
(4) Enantioselective recognition

In 2019 we used the ligand-exchange strategy to fabricate the first example of circularly polarized luminescent ZIFs. We chose 4-(10-phenylanthrancen-9-yl)phenyl and functional imidazole groups (R-/S-1) as chiral emitters. After blending with one kind of Zn-based ZIF (ZIF-8) nanoparticles, ligand-exchange happened and the glum was amplified from 7.0 × 10-4 to 5.5 × 10-3. When the ZIF-8 mixed with the a-hydroxycarboxylic acid (e.g. tartaric acid (TA)), fluorescence would be enhanced. More interestingly, the fluorescence of R-ZIF with L-TA showed a higher enhancement than D-TA, while the opposite trend in enantioselectivity was observed for the mixture of S-ZIF with TA. Two different diastereomeric complexes generated by the chiral sensors with the two enantiomers could be the reason for the different fluorescence enhancement. This phenomenon enabled enantioselective recognition.
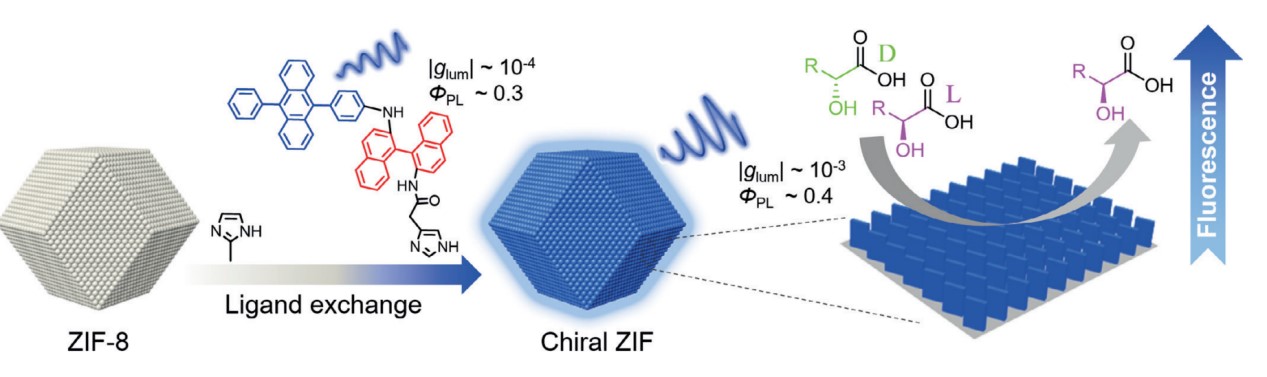
reference
Angew. Chem. Int. Ed, 2019, 58, 4978–4982
(5)Long-persistent circularly polarized phosphorescence

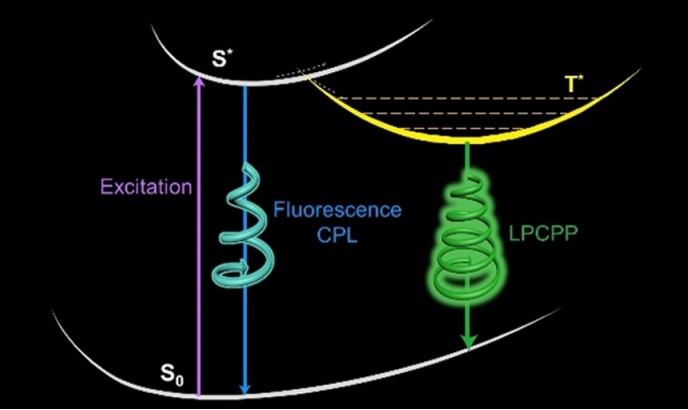
Phosphorescence, transmitting from triplet to singlet, typically has longer lifetime than fluorescence. However, Long-persistent circularly polarized phosphorescence (LPCPP) is not that common to see. In this work, we designed and incubated a pair of chiral organic ionic crystals, terephtalic (R/S)-phenylethyamine(TPA-(R/S)-PEA), which generated LPCPP. This ionic crystals showed two phosphorescence peaks at 425nm and 500nm, which generated from two different triplet states and had lifetimes of 2.11ms and 862ms. The large glum was measured at 500nm ( 1.5×10-2 and 2.0×10-2 in TPA-(R)-PEA and TPA-(S)-PEA, respectively). The achiral TPA molecules possessed chiral structures with a mirror relationship in co-crystals with PEA. The induced chirality of TPA structures may be responsible for the generation of chiral phosphorescence signals. In conclusion, this work had demonstrated the first example of long-persistent circularly polarized phosphorescence in organic ionic crystals.
reference
Chem. Eur. J, 2018, 24, 17444-17448